Light microscopy
European regulation EC/152/2009 (consolidated) and its associated SOPs state that official controls for the detection of PAPs in feed occur by light microscopy, except for aquafeed where PCR may also be used under defined conditions. The microscopic method is based on the identification of animal particles which can be detected by using both a stereomicroscope and a compound microscope. Typical structures such as bone spicules, cartilage fragments, muscle fibres, fish bones and scales, feather’s barbules or hairs can be detected by an experienced operator within plant and mineral matrices used for feeds.
Primarily before performing observations, feed samples are submitted to different treatments:
- Sieving or grinding in order to get particles of appropriate size
- Sedimentation in tetrachloroethylene (TCE) in order to separate the light components from the heavy ones. In general it allows separating most of plant material and other organic material from mineral ones – including animal bones.
- Double sedimentation (PE/TCE) for insect isolation.
- Different optional stainings intended to improve detection of certain constituents (e.g. Alizarin red colouring for bony material)
- Slide mounting with a suitable embedding medium.
Analyses have to be performed on raw sample material or flotate and sedimented material.
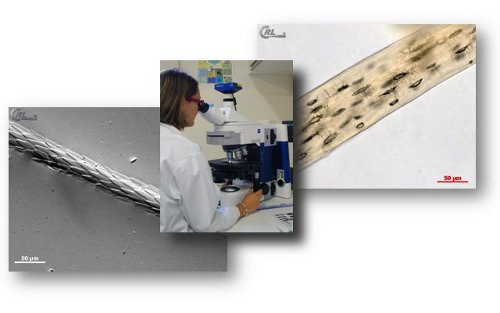
The method is qualitative. It delivers a result expressed as “present”, “absent” or “below the decision limit”. Based on morphological and histological features, the method enables to identify animal components from fish origin, terrestrial invertebrates (insects) and “terrestrial vertebrates” origin. The term terrestrial vertebrates embraces mammalian and bird animal remains which often present overlapping morphological criteria making them hardly distinguishable from each other. Nevertheless analysis from raw sample material will permit to detect, if present, feather fragments relevant for the presence bird material.
Advantages and drawbacks of light microscopy vs other methods are summarised in this document.
PCR (Polymerase Chain Reaction)
Biomolecular methods are complementary optimisations to the results obtained by light microscopy. Since publication of EU regulation 51/2013 amending EC/152/2009 PCR has become an official method for the detection of ruminant DNA in aquafeed.
Through genetic amplification, the PCR method enables well-defined taxonomic related DNA sequences to be detected: at species level (bovine, pig, chicken, and sheep) or at supra-species level (ruminants, fishes).
Basically the method involves the following steps.
- Extraction of the DNA from the feed.
- Multiplication of copies of the defined DNA targets (amplicons) by successive PCR thermal cycles. The number of copies is theoretically doubled at each cycle. Real time PCR allows following over the cycles –i.e. overtime– the increase of amplicon numbers by detection of the light emitted by a fluorescent DNA probe.
- Analyse of data referring to cycle threshold values and delivery of a qualitative result; it means a result expressed as “present” or “absent”.
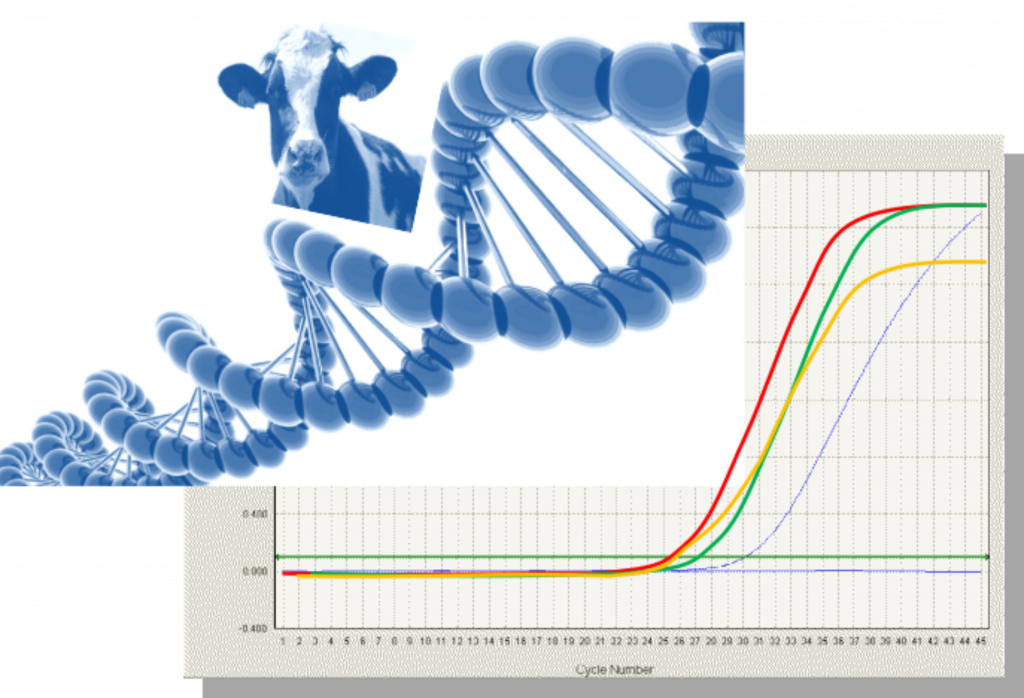
Kinetic of real time PCR is monitored while running on the thermocyclers. Several methods exists depending on the extraction mode, the differences among DNA targets for a given species (e.g. more than 10 different targets for porcine) and the type of equipment. Due to the drastic heating process of PAPs degrading the DNA, studies have demonstrated that in general these different methods succeeded at detecting bovine PAPs at levels of 0.1% in feed when the target chosen is a short amplicon (< 100pb) present in multicopy per cell.
Advantages and drawbacks of PCR vs other methods are summarised in this document.